David Drew
Structure and Mechanism of Solute Carrier (SLC) Transporters
SLC transporters are the targets for many therapeutics and they often play a major role in drug pharmacokinetics. Understanding the mechanisms by which SLC transporters shuttle and move ions, drugs, and natural compounds across membranes is of fundamental importance. Because of the technical difficulties in working with membrane proteins our mechanistic understanding is very limited. The Drew lab aims to understand the biophysical and chemical principles governing transporter function.
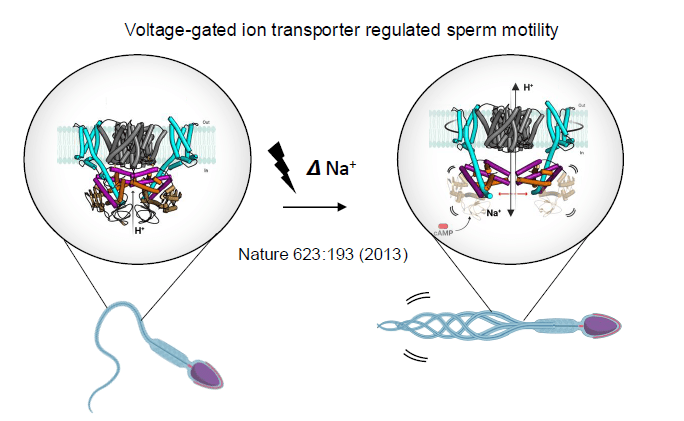
Solute transporters have to scan the cells surface to absorb only specific molecules out of all of the tens of millions of molecules they encounter. The Drew lab determined the first X-ray structures of a glucose (GLUT) transporter with doors open to both the outside and the inside of the cell. Drew has proposed a glucose transport mechanism and this model has been further refined by structures of a related malarial parasite sugar transporter with doors closed to both sides. GLUT transporters are responsible for maintaining blood glucose homeostasis in humans, and this work provides a foundation for their selective pharmacological control to combat human diseases, such as cancer and diabetes. His lab has further investigated how salt (sodium) is absorbed and exchanged for protons across cell membranes to control the cell’s internal pH by determining the first cryo-EM structures of mammalian Na+/H+ exchangers. These proteins operate very differently to the sugar transporters, and this work has aided in the general acceptance of an “elevator” transport mechanism, as first described in glutamate transporters.
The Drew lab will continue to combine cryo EM structures with biophysical and biochemical assays to deepen the mechanistic models for sugar and ion transport, and to establish how the activity of these proteins are regulated by interaction with other proteins and lipids. These goals are facilitated by the development of novel methods to aid in the functional and structural investigation of SLC transporters.
Funding
Knut och Alice Wallenberg, VR, ERC, Cancerfonden, Göran Gustafsson Foundation
Lab members
Senior Scientists
Ashutosh Gulati
Albert Suades
PostDocs
Jakob Silberberg
Jianan Chen
Hang Li
PhD students
Surabhi Kokane
Sukkyeong Jung
Lab manager
Magnus Claesson
