New SciLifeLab-developed method: an important step towards safer and more effective drugs
A team of SciLifeLab researchers have developed a new method called CeTEAM. This method was recently published in Nature Communications and will help reveal how drugs work in their target cells. Specifically, if a drug binds its target protein and if it has the desired effect. The approach was shown with cancer drugs, such as inhibitors of the DNA repair protein, PARP1, to show how some may trap it to DNA. Which can be critical to their clinical efficacy. In addition, the method was used to show how some drugs targeting the enzymes MTH1 and NUDT15 might cause unintended effects. It could prove invaluable for researchers trying to discover new drugs and to evaluate the most promising candidates.
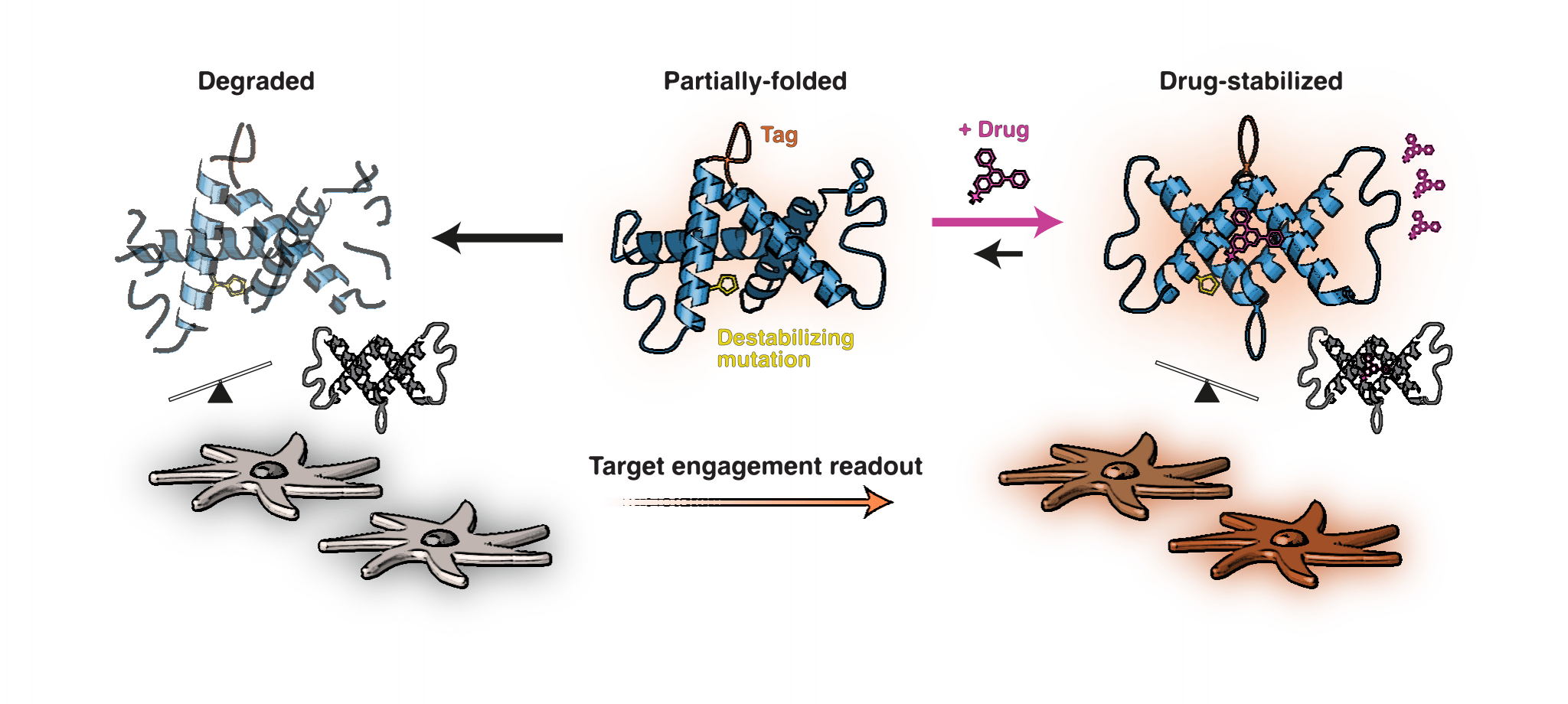
CeTEAM could be a transformative way to evaluate drugs in biological systems. In the past, researchers could tell if a drug was finding its target in a cell. However, it was difficult to determine how this relates to a drug’s action. This method fills that gap. Showing not only where drugs bind but also how this relates to its effects on the cell. This is an important step toward making drugs both safer and more effective.
The study was led by Nicholas Valerie and resulted from close a collaboration between the groups of Mikael Altun and Thomas Helleday at Karolinska Institutet and SciLifeLab, who performed most of the work. Drug library screening was facilitated by the SciLifeLab Compound Center infrastructure, which is part of the Chemical Biology Consortium Sweden (CBCS). The labs of Pål Stenmark (Stockholm University) and John Pascal (University of Montreal) also contributed insights into how the selected drugs bind to their targets.
One interesting finding is that suitable protein variants for CeTEAM might be more common than originally thought. For example, a compatible mutation in one protein may also work in closely related proteins. These mutant proteins, which may occur naturally or are discovered experimentally, coincidentally make it easier to observe drugs binding to their target in cells, so this discovery could expand the use of CeTEAM to many more drug targets in the future.
Here’s how the method works
When a drug binds to a protein, it often stabilizes the protein. But detecting this change in living cells isn’t easy. The researchers identified specific protein variants that highlight these changes, making them easier to study. Importantly, the method doesn’t interfere with the cell’s natural processes. Making it possible to measure the binding of a drug and its effects on the cells at the same time. It’s also flexible and can be scaled up, meaning researchers can test many drugs at once or study them in more complex systems.
“CeTEAM allows us to see more completely how drugs interact with their targets in live cells. For example, we can see if a drug’s effects are related to binding its intended target. So-called on-target and off-target effects. Which are crucial for understanding how it works and might benefit patients,” says SciLifeLab researcher and KI assistant professor Nicholas Valerie. SciLifeLab and KI researcher Mikael Altun adds: “This approach also makes it possible to screen many drugs at once. So we get a “birds eye view” of how certain types of drugs work overall. But also can be useful for non-invasive detection of drug binding in preclinical models, providing a better understanding of how drugs work in living systems.”
What’s next?
Looking ahead, the researchers plan to refine the method and expand its potential applications. They want to figure out which protein variants are the most useful and apply the method to different types of drugs. And explore how it could help pinpoint drug selectivity, ensuring drugs target the right proteins while avoiding others.
“We’re excited to see if these advancements could lead to better treatments for a range of diseases and even help with developing new types of drugs. Like those that break down harmful proteins. To facilitate new therapeutic modalities reaching the clinical stage”, says Valerie.
DOI: 10.1038/s41467-024-54415-7
Contact information
Nicholas Valerie, Assistant Professor
Laboratory Medicine
nicholas.valerie@ki.se
0706449051