New method for profiling complex biological dynamics at the single-molecule level developed: “broad applications across molecular biology, genetics, and drug discovery”
The MUSCLE method combines single-molecule fluorescence microscopy with next-generation sequencing, and makes it possible to profile the single-molecule dynamics of large numbers of different samples in parallel.
A collaboration led by SciLifeLab and Uppsala University researcher Prof. Sebastian Deindl has developed a method that vastly improves the ability to observe and analyze complex biological processes at the single-molecule level. Their work, titled MUSCLE: MUltiplexed Single-molecule Characterization at the Library scalE, is published in the journal Science.
The new method overcomes a significant limitation in the field of single-molecule fluorescence microscopy, which, until now, has been restricted by low throughput. Traditional approaches have been limited to studying a small number of representative samples, often leading to biases and missed opportunities for discovering novel insights within large libraries of molecules.
MUSCLE addresses this challenge by combining the mechanistic insights from single-molecule fluorescence microscopy with the high throughput capabilities of next-generation sequencing.
Profiling large numbers of distinct samples in parallel
The MUSCLE workflow begins by attaching a library of fluorescently labeled molecules onto a surface known as an Illumina MiSeq flow cell. This flow cell is then placed onto a single-molecule fluorescence microscope using a 3D-printed adapter, allowing researchers to observe the real-time dynamics of individual molecules across multiple fields of view.
After imaging, the flow cell is subjected to standard Illumina sequencing, which generates clusters of identical copies from the molecules previously observed. These clusters are then matched with the corresponding molecules based on their positions on the flow cell.
“Spatially registering the single-molecule imaging and Illumina sequencing data turned out to be extremely challenging, but careful detective work to figure out the coordinate system used by the Illumina instrument allowed us to solve this problem” says Dr. Anton Sabantsev.
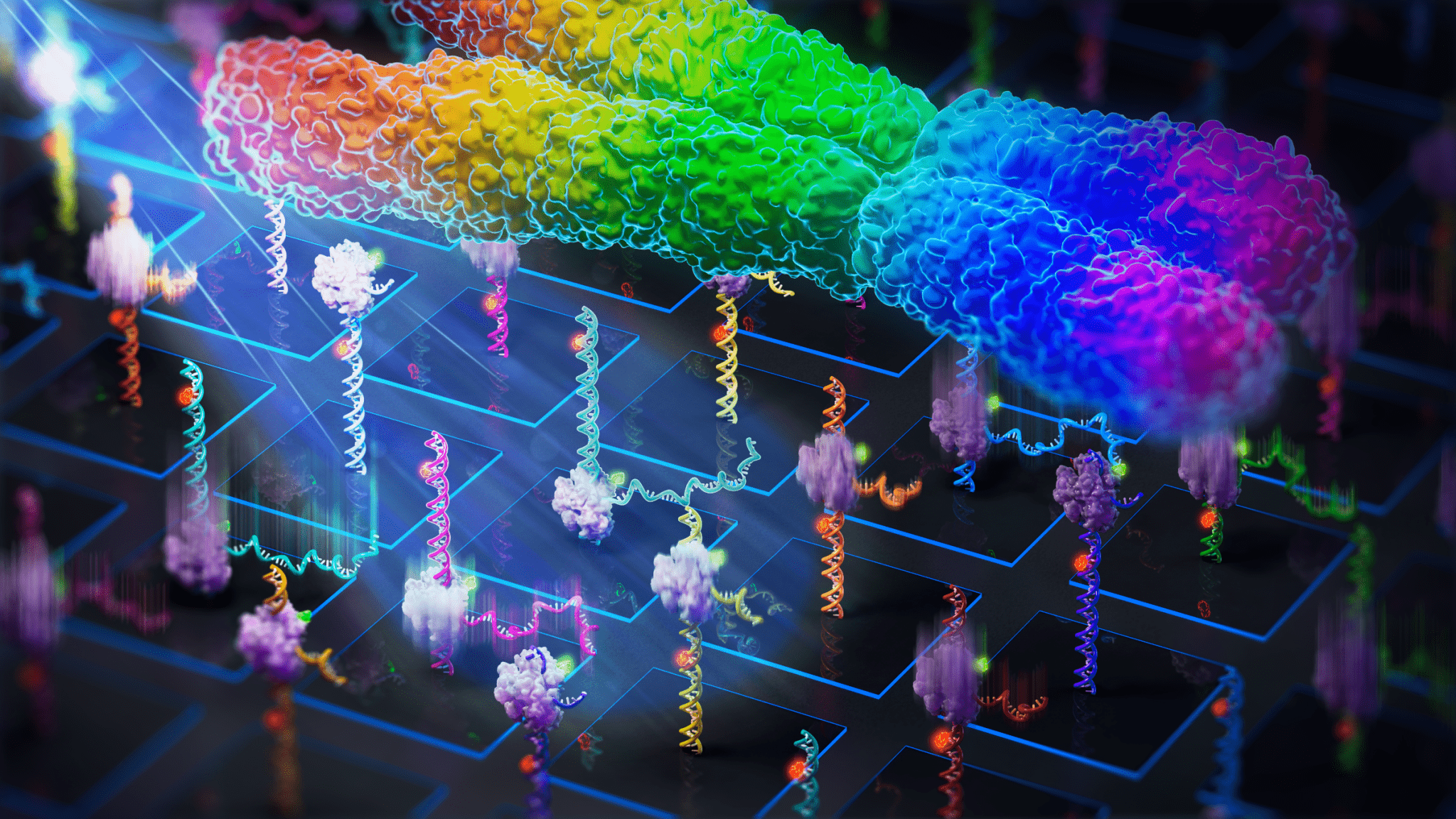
This innovative approach allows researchers to simultaneously profile the dynamics of a vast array of sequences, providing a more comprehensive understanding of complex biological processes.
“Our method allows for the direct observation of dynamic molecular behaviors across extensive libraries, significantly enhancing our ability to uncover general trends, outlier behaviors, and unique dynamic signatures that would otherwise remain hidden” says Sebastian Deindl, senior author of the study. “MUSCLE transforms how we study the intricate dynamics of biomolecules, with broad applications across molecular biology, genetics, and drug discovery.”
Teamwork essential
“Key to this very challenging multi-year effort was the wonderful teamwork among the members of our group. Everyone brought something different to the table, which was crucial for overcoming the technical hurdles we faced” says Dr. Guanzhong Mao, one of the co-first authors, together with Dr. Javier Aguirre Rivera, Dr. Anton Sabantsev and Mikhail Panfilov.
The research team also included Magnus Lindell from the SciLifeLab National Genomics Infrastructure in Uppsala, who facilitated the next-generation sequencing into the MUSCLE workflow.
Democratization of advanced methods
Given its reliance on widely available fluorescence microscopy and MiSeq instruments, along with the ease of fabricating the necessary adapter using 3D printing, the MUSCLE method is highly accessible to the broader scientific community. It can be adapted to study a wide range of proteins interacting with nucleic acids, as well as DNA-barcoded proteins, compounds, or ligands.
“This breakthrough opens the door to more accurate and comprehensive studies of biological systems, where understanding the full spectrum of molecular behavior is critical. The MUSCLE method is expected to have a profound impact on the study of complex molecular dynamics as a function of sequence or chemical space, enabling researchers to explore previously uncharted territories in biology” says Sebastian Deindl.
The article can be found here: https://www.science.org/doi/10.1126/science.adn5371
Top photograph by Mikael Wallerstedt.

Sebastian Deindl, Ph.D.
SciLifeLab researcher
Professor of Molecular Biophysics
Wallenberg Academy Fellow
Department of Cell and Molecular Biology
Uppsala University
Phone: +46 (0) 70 840 0496
Email: sebastian.deindl@icm.uu.se
Web: http://deindl-lab.com