Ilaria Testa aims to observe every molecule
She developed a microscope that cut the time of imaging a living cell with molecular resolution from about forty minutes to half a second. Now Ilaria Testa wants to shed light on neuronal processes by observing morphology that no one has ever seen before.
Ilaria Testa works, as she puts it, “at the interface between physics and chemistry” – on the one hand greatly improving microscopes to measure finer details at greater speeds, and on the other being interested in fluorescent molecules to better use those microscopes. She also applies the methodological and technological advances. The super resolution microscopes her group develops makes answering intricate biological questions very appealing.
“I have recently started to develop an interest for how basic molecular mechanisms inside neurons work”, says Ilaria Testa.
Beyond the physical limits
For centuries, scientists have thought that light microscopy has a limit in how small things it can magnify. Lenses in light microscopes focus the light to a specific point. Because light bends, you can only focus it to a certain size – about a hundred times bigger than a molecule. This is called the diffraction limit. The idea has been that since you cannot focus the light smaller than that, looking at objects even tinier is not, and will never be, possible with light microscopy. Nowadays, it’s a reality – thanks to the field Super-Resolution Light Microscopy and scientists like Ilaria Testa.
A couple of decades ago, researchers started to think about light microscopy from a different perspective. Instead of focusing light to a point, could it be possible to color the molecule with fluorescence that can be switched on and off to make the observed region in the sample smaller? It turned out that this is, in fact, possible. In those few decades, the field has gone from a strictly theoretical concept to actual microscopes approaching a nanoscale and looking at living cells in 3D.
Why anyone would bother with this when electron microscopes can already do it is, according to Ilaria Testa, because in contrast to electron microscopes, super-resolution light microscopes can visualize living cells.
“We can follow the process over time, we can view it in 3D and we can look at specific molecules. With electron microscopes, most molecules are the same, it’s more about morphology where you distinguish philaments from membranes, but you don’t know exactly what you are looking at”, says Ilaria Testa.

The MoNaLISA
So, where is Ilaria Testa in all of this? Well, she has been researching microscopes since her Bachelor’s thesis in the early 2000s. From having studied quantum physics she was hooked on fluorescent molecules and microscopy at first glance.
“The first time I saw a single molecule popping up was amazing. I will never forget it, I wanted to do that for the rest of my life”, says Ilaria Testa.
In 2018 she developed the MoNaLISA microscope – short for Molecular Nanoscale Live Imaging with Sectioning Ability. When starting the MoNaLISA project, Ilaria Testa and her group had microscopes that were working well with fixed cells, but lacked a technique that would produce the same quality in living, behaving, cells.
“To do this, the microscope does not only have to be good at recording spatial detail, but also fast enough when the cell is moving”, says Ilaria Testa.
In the MoNaLISA microscope, a certain fluorescent molecule that can switch on and off its light emission many times is used. The first step in developing the microscope was therefore to find a molecule that could do that, but at the same time could be expressed in living cells. The switching can basically be described as a molecule being able to exist in two states – one where it is detectable and one where it is not. This is done with light, so it does not need to be touched, illuminating the molecule forces it to switch between the two states.
A donut shaped light is switching on a small part of the molecules, which is recorded. As the donut moves over the structure, it records all molecules step by step. An image of the whole cell in very high detail is then constructed from all of the parts. A donut light was already used in method stimulated emission depletion (STED), a technique that won the Nobel prize in 2014. What is new in the MoNaLISA, to name one thing, is that instead of using one donut it utilizes more than 4 000 holes acting in parallel – something called periodic line pattern and multi spots. This has cut the time of recording a whole cell from about forty minutes to half a second. Another novelty in the MoNaLISA is a biochemical switching mechanism called cis-trans isomerization which uses a lower illumination dose – that is compatible with living cells.
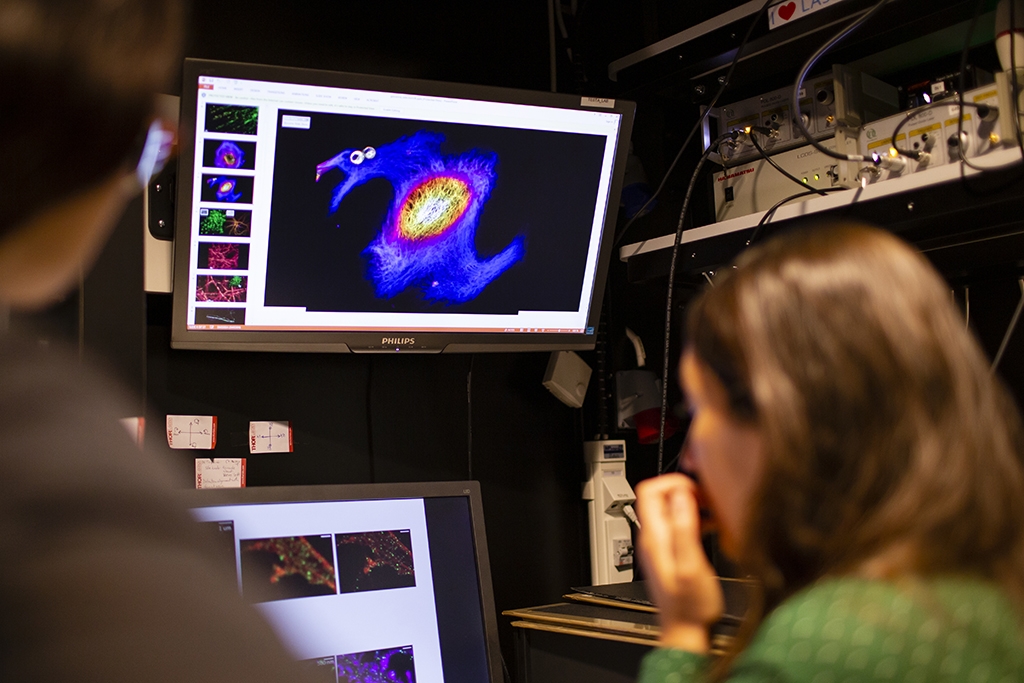
“So now we have an instrument that people have been using for a long time, like a confocal microscope, but with ten times better resolution, with which you can look at smaller things in very crowded environments, like the synapse, and are able to ask better questions”, says Ilaria Testa.
With the help from sharks
Switching molecules on and off is harder than it may sound. Until recently, no one really knew what molecules to use in this type of application. Ilaria Testa and her group have worked hard to find a few that works the way they need them to.
When imaging, the fluorescent molecules have to be delivered to the structure in some way. A common way to do this is with antibodies, which are specialized in finding specific things in the body. The problem with antibodies, however, is that they are big – relatively speaking, they are still super small. The antibody sits between the fluorescence and the structure, what you see is thus an area close to the structure, not the structure itself. To be able to get closer, and thereby getting images with greater detail, Ilaria Testa had to find smaller molecules capable of delivering the fluorescent molecules. What they used was teeny tiny molecules from alpacas and sharks.
“They are smaller compared to antibodies and you don’t necessarily have to kill the animal to get them, you can take a little bit and they can still specifically bind to the molecule of interest”, says Ilaria Testa.

Developing new ways to deliver the fluorescence is a whole other field, not something they specialize in at the Testa lab, but it is a big part of how successful their microscopes can get so they need to stay up to date on what is discovered, and above all: Try different things to see what works.
“We are often asking people about this: “can we try your switchable molecules?”, “can we try CRISPR?”, “can we try this and see how it works in our microscope?”, because sometimes the physics is there, but you need better labeling to arrive at the scale you need to better see a structure”, says Ilaria Testa.
The perfect fit
The person that got Ilaria Testa into physics was her math teacher. A charismatic personality that always mentioned quantum mechanics: Rules that are not intuitive but in a way end up explaining parts of this world, intriguing to say the least.
“This was a big inspiration for me. I wanted to better understand what we do, where we live, and then physics is the answer”, says Ilaria testa.
Her interest in fluorescent molecules and microscopes took her from the University of Genoa, via the Max Planck Institute, and eventually to SciLifeLab. She was always scared of choosing between universities and research institutes, thus closing the door to some aspects of research. On the one hand, she wanted to do excellent research, but on the other, she found meeting and forming students to be extremely important. SciLifeLab was the perfect fit.
“Here was this opportunity to belong to KTH, a technical university that would understand the importance of technical development, and at the same time a research institute that would understand what the everyday challenges in biology and biomedical research are. SciLifeLab was both of them”, says Ilaria Testa.
You can tell that Ilaria Testa is taking her supervising role seriously. She talks passionately about how you need to be flexible in the kind of leader you are: Sometimes you need to step in and delegate, but you also need to let your younglings test their wings.
“There’s a time when you want to create a certain culture in the lab and get everyone on the same page of what you think is important, but then you also need to leave them free to come up with their own ideas and support them”, says Ilara Testa.

She lives and breathes imaging, her biggest interest outside of work is not terribly shocking: photography. It is not macro photography that grabs here attention though, black and white portraits do.
“It minimizes the boundaries between people in a way, you get closer to a certain shade of a person. Lately I’ve also been more into animals, and seeing if I can catch a certain activity or communicational behavior. And of course also the technology, I’ve got a deeper understanding of camera technology”, says Ilaria Testa.
Inspired by reality
At work, technology development is still very much on the agenda for Ilaria Testa, but she is also interested in using what they have accomplished tech wise to answer complex biological questions – about neuronal processes at the moment.
“I think the best way to push technology forward is being inspired by a question”, says Ilaria Testa.
One ongoing study investigates how binding proteins behave in synapses – how molecules are relocated when neurons are firing and what other molecules are involved. Another study is observing how the neuronal mitochondria change morphology in order to better fuel the synapse and allow the synaptic process to actually happen.

Ilaria Testa is interested in connecting the fine morphology of the mitochondria with their function. With the MoNaLISA, they can observe the morphology in a way that no one has ever seen before. From that they aim to develop new theory and move on to questions of more functional interest.
“So why mitochondria? Because mitochondria are very important for the neuronal homeostasis, for the neuron to function like a neuron, without mitochondria that wouldn’t happen”, says Ilaria Testa.
Another reason they focus on the mitochondria is that people suffering from neurodegenerative diseases have a lot of mitochondria related mutations. They have for example found that mitochondrial structures come close to each other, connect and then retract. Ilaria Testa is curious about what the mitochondria are transporting, why they decide to connect and exchange materials.
“Is it more about mitochondria communicating with the rest of the cell or is it about themselves and their internal machinery to work properly? Why are they there? And why do they assume these shapes? It’s just new, no one has ever seen this level of detail in living neurons before, so you have a lot of questions, the challenge is to focus on the right one and solve one after the other”, says Ilaria Testa.
Capturing the living
There are several challenges when imaging living cells. One of these is the characteristics of the molecule delivering the fluorescence. If you want to see something inside the cell, the molecule needs to be small enough to go through the membrane of the cell without making a hole – otherwise the cell dies, and you are obviously not imaging a living cell anymore. Antibodies, for example, are too big. Finding fluorescent proteins with the right characteristics is therefore important.
“We started with zero proteins but we now have a few, we are building a library”, says Ilaria Testa.
Another challenge is that the illumination needs to be cell compatible. When you look at dead cells you can go nuts with the energy of the light, you do not risk killing the cell because it is already dead. With living cells, however, phototoxicity is a real issue. Less energy equals lower probability of damage to the cell. Some biochemical switching mechanisms use less energy, but the color of the light is also important – red light is less energetic but makes it harder to get a good result, for example.
The most important challenge when imaging living cells, according to Ilaria Testa, is speed, however. A fact that is quite intuitive when you think about it, if something moves when you are taking a photo the result risk being less than optimal if the shutter speed is not fast enough. When imaging cells, some processes are slower than others. In some, an hour of recording is enough. Others – like synaptic vesicle endocytosis – happen in milliseconds.

A saying the Testa lab lives by is “do not waste time in dark pixels”. What they mean by this is that an image consists of a lot of space that is not relevant – and is literally dark. They therefore developed a feedback system – called smartRESOLFT – which allows the microscope to go really fast over parts that are not relevant to record. A simple idea, but harder to implement in practice – you need advanced electronics, a cross-feedback loop and the know-how.
“Right now we have a technique that can take about fifteen hundred frames in real time, 24-30 frames per second, and this is already fast enough to look at for example mitochondrial dynamics or peroxisome organelle dynamics. Four years ago that was not possible, there was probably one frame and the rest was bleached. That’s another thing about fluorescent molecules, they’re not forever, so it’s always important to use new probes.”, says Ilaria Testa.
Could you describe every molecule?
“Now we can look at one molecule at a time, maybe two, maybe in a very good case four, but we will develop more tools and finally describe them all”, says Ilaria Testa.
Read more at the Ilaria Testa research group page: https://scilifelab.se/researchers/ilaria-testa/
Text & Photo: Niklas Norberg Wirtén