A new flavor of heterochromatin
SciLifeLab researchers have discovered a new ‘flavor’ of heterochromatin by studying how mouse embryonic stem cells can suppress the mobilization of ‘parasitic’ transposable elements. Their finding questions some of the fundamental principles of heterochromatin.
Histones act as key players in the most fundamental layer of gene regulation. Euchromatin is characterized by a loose packaging of the chromatin, allowing transcription machinery to access the underlying DNA and express the contained genes. Heterochromatin is a fundamentally distinct compartment characterized by a compacted, inert chromatin fiber, rendering the underlying DNA inaccessible.
Heterochromatin protects the cell from untimely expression of genes and also aberrant expression of abundant ‘junk’ DNA consisting of repetitive sequences from viruses and endogenous ‘parasitic’, transposable DNA elements. Protective mechanisms like this can fail in ageing cells or cancer cells, triggering a mobilization of transposons with relatively unknown consequences.
Methylation of the DNA and histone proteins (most prominently histone H3 lysine 9 trimethylation, H3K9me3) are key posttranslational modifications that lead to chromatin compaction.
In a recent study, published in Nature Communications, a team of SciLifeLab researchers, led by Fellow Simon Elsässer (Karolinska Institutet), discovered a new ‘flavor’ of heterochromatin that questions some fundamental principles of heterochromatin.
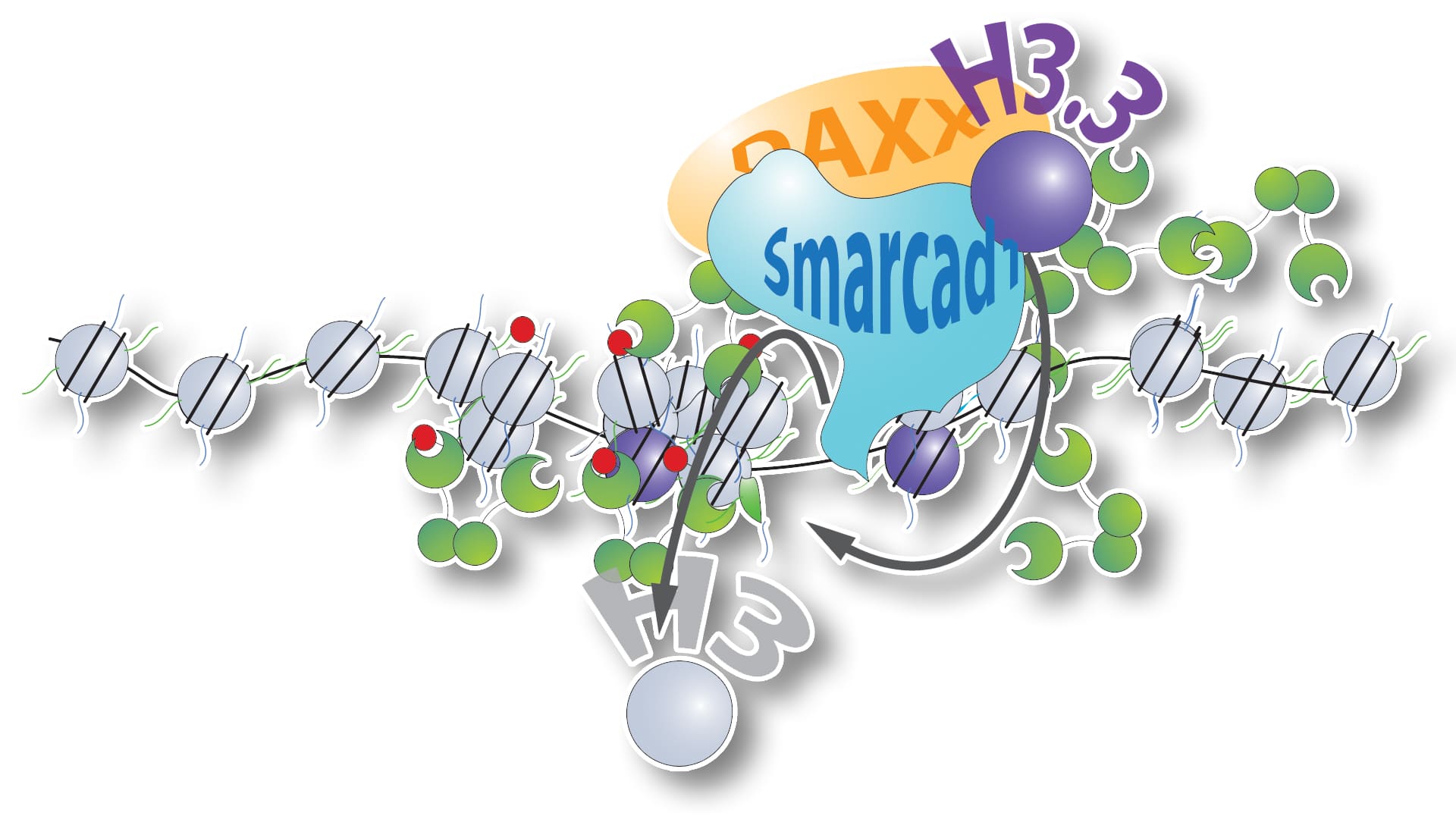
Studying the mechanism that mouse embryonic stem cells employ to suppress the mobilization transposable elements, they found that heterochromatin formed over these transposons showed strong signatures of dynamic histone exchange despite exhibiting all key features of canonical heterochromatin.
Exchange of histones has been thought to be an exclusive feature of euchromatin since it requires the nucleosome to unwrap, making the DNA accessible. Surprisingly, the team uncovered a mechanism that uses ATP to swap existing histones for new histones within the tightly compacted heterochromatin domain.
“The concerted process of eviction of one and deposition of another histone is so smooth and efficient that it leaves no apparent trace of accessible DNA. Without a close look at the dynamics of histones within the chromatin fiber, we would have never noticed the phenomenon”, says Simon Elsässer.
The results are puzzling since a constant replacement of histones should counteract a dense chromatin structure that is protected from transcriptional activation. However, the researchers believe that this ‘dynamic’ heterochromatin is an adaptation of a ubiquitous silencing mechanism to the specific requirements of a pluripotent chromatin state.
The very brief opening of the heterochromatin may allow sequence-specific co-repressors to bind to their target sequences within the transposable element, leading to a recruitment of more repressive factors to propagate and amplify the silent state.
Mutations and dysregulation of the factors involved, namely chromatin remodeler Smarcad1, histone chaperone DAXX and histone H3.3 have been observed in various cancer types. The new study raises the question if reactivation of silenced transposable elements may play a role in their tumorigenesis.