SciLifeLab collaboration leads to complete in silico GLUT5 cycle
Researchers from SciLifeLab have successfully constructed the most comprehensive transport cycle of a glucose (GLUT) transporter to date, and have determined the sensitivity of these proteins to lipids. These findings hold significant importance for a fundamental mechanism in human physiology.
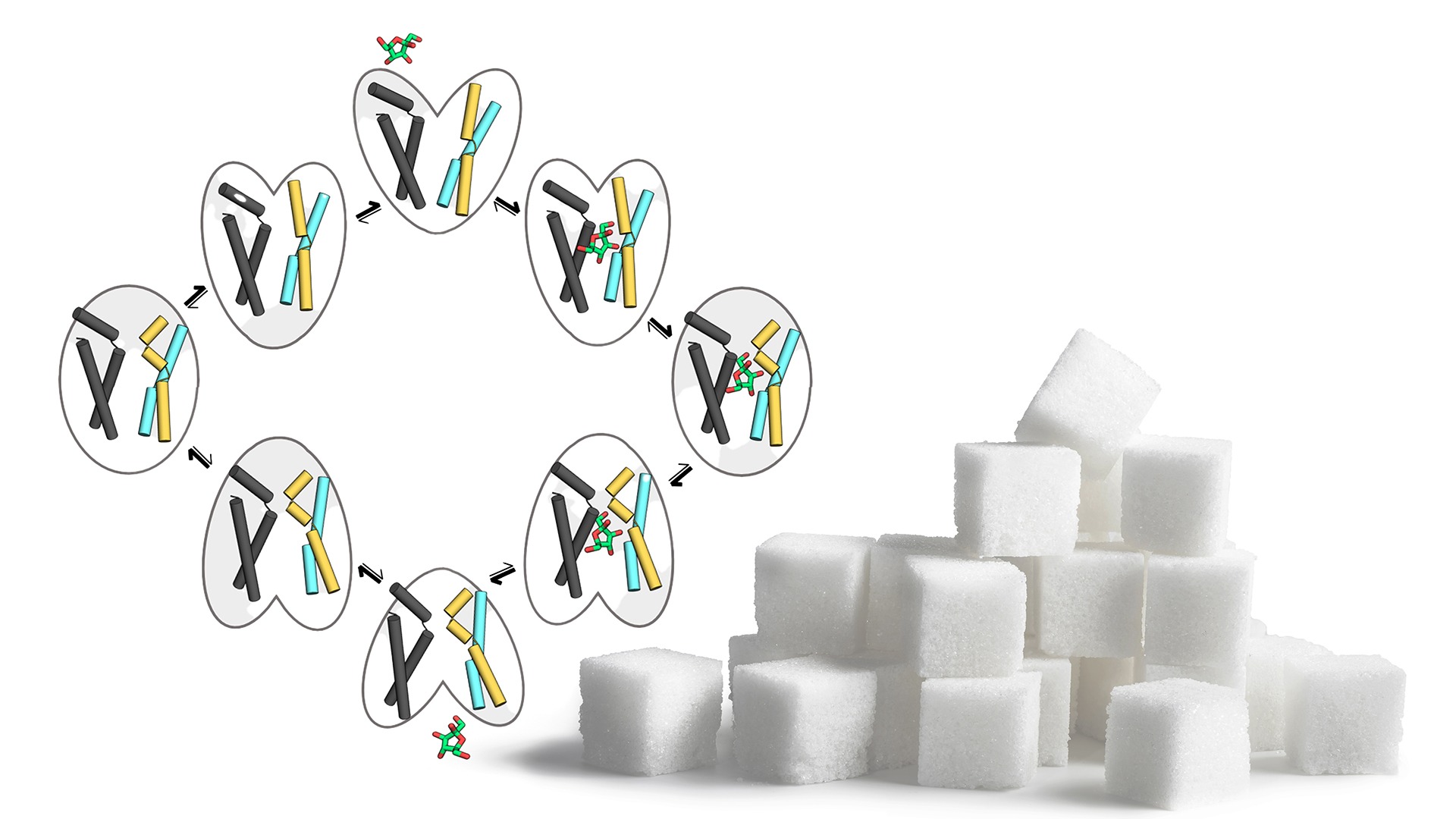
Simple carbohydrates, such as glucose and fructose, serve as important sources of energy for our cells, and in order for our cells to be able to use this energy, the carbohydrates need to be transported into the cells. This import task is carried out by proteins known as GLUT transporters, which act like doors and make it possible for the carbohydrates to pass through the membrane of our cells. GLUT transporters cycle through five major conformation states to transport a single sugar molecule and they are highly selective regarding which sugar to transport, despite binding sugar molecule with weak affinities. By using a computational approach and testing their model with biochemical assays, SciLifeLab researcher David Drew (SU) and SciLifeLab Fellow Lucie Delemotte (KTH) have been able to construct a complete in silico transport cycle for the fructose-specific isoform GLUT5, for the first time.
“It took us around eight years to obtain the crystal structures of two major GLUT5-conformations and then a further four years to capture an intermediate (occluded) conformation, but this was from a homologue found in the malarial parasite (Nature 526:394; Nature 578:31). By teaming up with Lucie Delemotte we have finally been able to combine all these static crystal structures into a comprehensive transport cycle to understand how sugars are able to catalyze their own influx. In essence, we can show that the transition state of soluble enzymes is the equivalent to the occluded state of membrane-bound transporters, except that the binding energy is used to drive the conformational change required for transport”, explains David Drew.
In order to better understand the mechanism of sugar transport in the entire sugar porter family, the two labs further trained a neural network, based on the available structures of family members and co-evolution analysis, to generate models of all sugar transporters in all different conformational states.
“By comparing these various models and scrutinizing their sequence, we have been able to define the aspects of the transport cycle that are conserved throughout the sugar porter superfamily and which may be specific to a given family member. This type of family-wide work is enabled by high-quality publicly available data and is part of an increased trend of using data-driven approaches to substantially broaden our insights”, says Lucie Delemotte.
Lastly, GLUT transporters have been studied for decades, but the measured rates for glucose transport in cells vary tremendously, and often the non-physiological sugars that has been used instead of glucose are rapidly metabolized.
“We were frustrated that GLUT transporter activities in human are described largely by their Michaels-constant (KM), but their rates are just as important to understand as this tells us what their top-speed is”, Drew explains.
By establishing a transport assay with purified GLUT proteins, the researchers were able to measure both these rates, but with a few surprises. For instance, while the insulin-regulated GLUT4 transporter exhibits a comparable affinity to the ubiquitous GLUT1, it demonstrates a considerably slower turnover rate. This disparity suggests a potential form of intrinsic regulation, ensuring that glucose uptake into fat and muscle cells is controlled by insulin-dependent trafficking. Furthermore, GLUT transporters are highly sensitive to the stiffness of the membrane, its fluidity, and its likely high levels of free fatty acids, found in individuals with metabolic disorders. These factors could directly impair GLUT4 activity by influencing membrane fluidity.
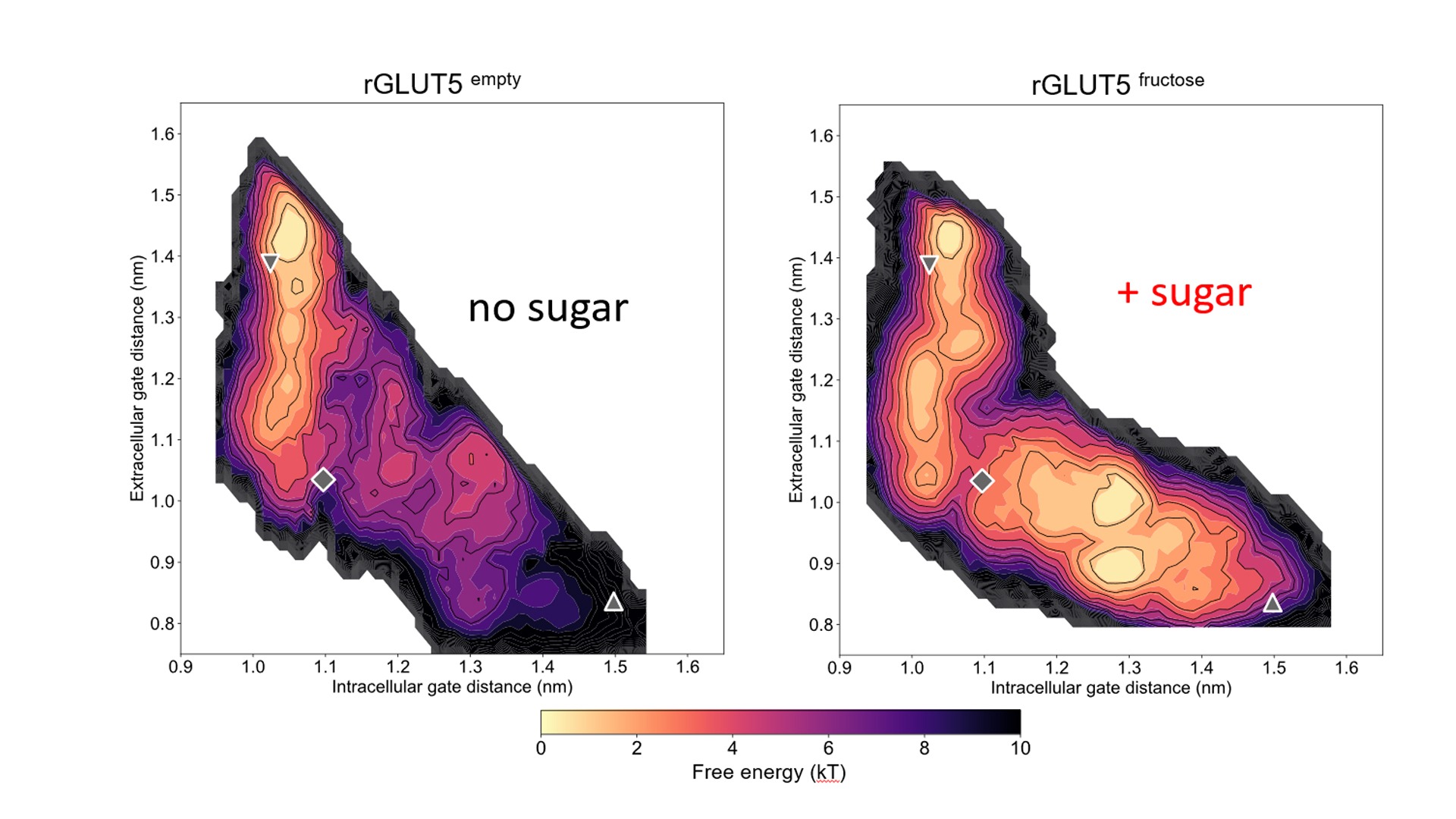
Understanding the molecular basis for sugar transport and their regulation opens up new avenues for pharmacological control, as these proteins are upregulated in metabolic disorders and many types of cancer.
To read the three publications, use the following links:
eLife 1 (2023)
eLife 2 (2023)
Nature Communications (2023)